Lester C. Geonzon1*,**, Motoyoshi Kobayashi1, Kaede Takatsuno2, Shingo Matsukawa2
Gelatin is a highly versatile hydrocolloid that plays a significant role in
various applications as both a gelling and emulsifying agent in the food and
pharmaceutical sectors. In the food industry, the mechanisms by which
gelatin stabilizes emulsions have been extensively studied. Nonetheless, the
dynamic changes, specifically the reconfiguration of the gelatin layer at
the interface when pH changes occur in situ, such as during gastric transit,
are frequently overlooked. In this study, we aimed to establish the
relationship between dynamic pH changes and the structural reconfiguration
of adsorbed gelatin on a model colloidal particle using microfluidics and
optical tweezers. Microfluidics allows for variation in the solution
environment, while optical tweezers enable the measurement of the
hydrodynamic layer thickness of adsorbed gelatin in the presence of a flow
field. When a 50 ppm gelatin solution prepared at pH 8.5 (isoelectric point)
was injected, a temporal increase in the hydrodynamic layer thickness was
observed (Fig. 1), indicating the adsorption of gelatin. Moreover, when a
low pH solution was injected, an increase in the hydrodynamic layer
thickness was demonstrated. This suggests a pronounced swelling of the
gelatin layer at the interface, which is attributed to an increase in the
net positive charge density, enhancing electrostatic repulsion between the
adsorbed polymer chains. When the solution pH was changed back to high pH, a
decrease back to the original adsorbed layer thickness was observed. Thus,
this study provides important insights into the structural reconfiguration
of the adsorbed gelatin onto a single colloidal interface.
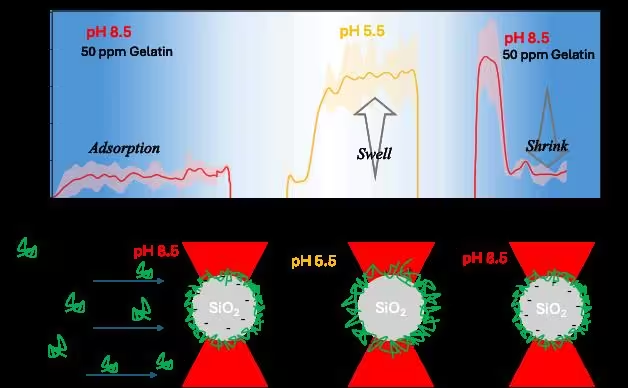 Fig. 1. Development of layer thickness during adsorption and changing pH conditions
Fig. 1. Development of layer thickness during adsorption and changing pH conditions