1. Introduction
Pickering emulsions stabilized by plant derived particles align with clean
label demands yet faba bean protein isolate (FBPI) often shows limited
interfacial performance due to low solubility and compact globular
structure. Non covalent interactions with polyphenols can modulate protein
conformation but the cooperative effects of dual polyphenols remain
insufficiently understood. This study aimed to enhance Pickering emulsion
stability by forming FBPI complex nanoparticles with gallic acid (GA) as a
small phenolic acid and rutin (RU) as a bulky flavonoid glycoside. The
objective was to clarify how the complementary molecular characteristics of
GA and RU generate cooperative structural and functional modulation of FBPI,
thereby reshaping its molecular conformation, particle level properties, and
interfacial assembly within a moderately unstable emulsion system with an
oil volume fraction of 0.6.
2. Materials and Methods
Pretreated FBPI was mixed with GA at a fixed concentration followed by RU
addition at increasing RU to GA ratios (0.0:1.0 ~ 1.0:1.0). Non covalent
FBPI-RU/GA complex nanoparticles were characterized for particle size,
ζ-potential, polyphenol binding content, solubility, exposed sulfhydryl (SH)
and amino residues, and secondary structure. Pickering emulsions were
prepared using canola oil and analyzed for droplet size (D4,3),
ζ-potential, interfacial protein adsorption, Turbiscan stability index
(TSI), and microscopic features. Statistical differences were determined by
analysis of variance with Duncan test at p < 0.05.
3. Results and Discussion
GA reduced FBPI particle size (Fig. 1A), increased solubility (Fig. 1C), and
transformed disordered secondary structures into more ordered α-helix and
β-sheet forms (Fig. 1F), improving dispersion and interfacial affinity.
Incorporation of RU progressively increased polyphenol binding (Fig. 1B) and
exposure of functional residues (Fig. 1D-E) while altering the balance
between compaction and unfolding. At an intermediate RU to GA ratio of 0.4
to 1.0, nanoparticles exhibited high solubility, more negative surface
charge, and a compact yet flexible secondary structure. These features
enabled efficient migration to the oil water interface and formation of
dense particulate films. The resulting emulsions showed smaller D4,3,
higher magnitude of ζ-potential (Fig. 1G), continuous fluorescent
interfacial rings in microscopy (Fig. 1K), and the lowest TSI (Fig. 1I),
reflecting strong resistance to coalescence and creaming. When RU proportion
exceeded the optimal level, over unfolding and steric congestion occurred,
reducing effective interfacial packing and weakening the particulate film.
These effects were evident in larger D4,3, decreased interfacial
protein concentration, and increased TSI values. Collectively these findings
indicate that Pickering stabilization depends on achieving an optimal
balance in FBPI structure where GA driven compaction and RU driven unfolding
act cooperatively rather than independently.
4. Conclusion
Dual polyphenol complexation offers a tunable route to engineer FBPI for
clean label Pickering emulsions. A GA RU ratio of 0.4 to 1.0 produced
nanoparticles forming cohesive and elastic interfacial films that markedly
enhanced stability, whereas excessive RU impaired assembly. This work
clarifies how dual polyphenols modulate FBPI and provides a design framework
for next generation plant-based stabilizers.
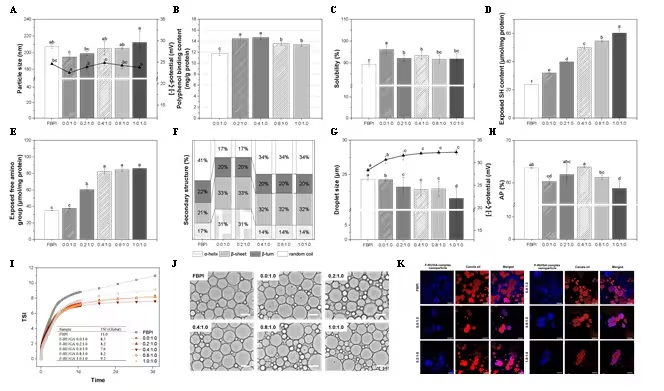 Fig. 1. Particle size and ζ-potential (A), polyphenol binding content (B), solubility (C), exposed SH group (D), exposed free amino group (E), and secondary structure of FBPI-RU/GA complex nanoparticle; droplet size and ζ-potential (G), adsorbed protein at the interface (H), Turbiscan stability index (I), optical microscopy images (J), and CLSM images of Pickering emulsions stabilized by FBPI-RU/GA complex nanoparticle. Scale bar=20 μm.
Fig. 1. Particle size and ζ-potential (A), polyphenol binding content (B), solubility (C), exposed SH group (D), exposed free amino group (E), and secondary structure of FBPI-RU/GA complex nanoparticle; droplet size and ζ-potential (G), adsorbed protein at the interface (H), Turbiscan stability index (I), optical microscopy images (J), and CLSM images of Pickering emulsions stabilized by FBPI-RU/GA complex nanoparticle. Scale bar=20 μm.