1128Effect of the molecular structure of the gelling agent on the gelation behavior of xyloglucan
1Dept. of Applied Bioscience, Kanagawa Institute of Technology, Atsugi, Kanagawa, Japan
Xyloglucan extracted from tamarind seeds is a complex, branched polysaccharide composed of a cellulosic backbone substituted at the O6 position with side chains of α-1,6-linked xyloses, 1,2-linked β-galactoses, and occasionally α-1,2-linked fucoses. Xyloglucan has significant potential for commercial applications, particularly in the pharmaceutical and food industries, where it can be used to control drug release and modify texture. Although this polymer does not form a gel on its own, it can exhibit gelation behavior when mixed with certain small molecules, such as iodine, Congo red, Eriochrome Black T, and gallate analogs. In the present study, the gelation behavior of xyloglucan in the presence of various molecules was investigated to elucidate the underlying gelation mechanism and to identify the molecular structures responsible for inducing xyloglucan gelation.
Initially, the gelation behavior of xyloglucan was examined using gallic acid (GA) as a gelling agent. A sol–gel transition was observed in a 3.0 wt% xyloglucan solution. The sol–gel transition temperature (gel-melting temperature) was determined by the falling-ball method. Xyloglucan and GA were fully dissolved in water in a 8-mm glass tube and allowed to gel at 4 °C. A steel ball was placed on top of the gel. Upon heating, the gel eventually melted and converted to a sol, at which point the steel ball began to move downward. The temperature at which the ball started to move was defined as the gel-melting temperature.
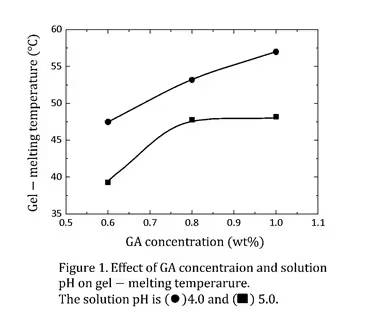 Figure 1 shows the effect of GA concentration and solution pH on the
gel-melting temperature. The gel-melting temperature increased with an
increase in GA concentration, reaching approximately 60 °C. Moreover, the
gel-melting temperature was higher at lower pH values (pH 4.0). Since the
pKa of GA is about 4.5, the fraction of protonated acid species is high at
pH 4.0, These results indicate that protonation of the carboxyl group plays
a crucial role in controlling gelation behavior. In addition, the molecular
structure of the gelling agent is critical for inducing xyloglucan gelation.
Replacement of the aromatic ring in GA with an aliphatic chain of the same
carbon number resulted in weakened gel strength. Furthermore, the relative
positions of the carboxyl and hydroxyl groups were found to be key factors
in determining the sol–gel transition temperature of xyloglucan
Figure 1 shows the effect of GA concentration and solution pH on the
gel-melting temperature. The gel-melting temperature increased with an
increase in GA concentration, reaching approximately 60 °C. Moreover, the
gel-melting temperature was higher at lower pH values (pH 4.0). Since the
pKa of GA is about 4.5, the fraction of protonated acid species is high at
pH 4.0, These results indicate that protonation of the carboxyl group plays
a crucial role in controlling gelation behavior. In addition, the molecular
structure of the gelling agent is critical for inducing xyloglucan gelation.
Replacement of the aromatic ring in GA with an aliphatic chain of the same
carbon number resulted in weakened gel strength. Furthermore, the relative
positions of the carboxyl and hydroxyl groups were found to be key factors
in determining the sol–gel transition temperature of xyloglucan
Physical properties of food hydrocolloids for enhanced product development