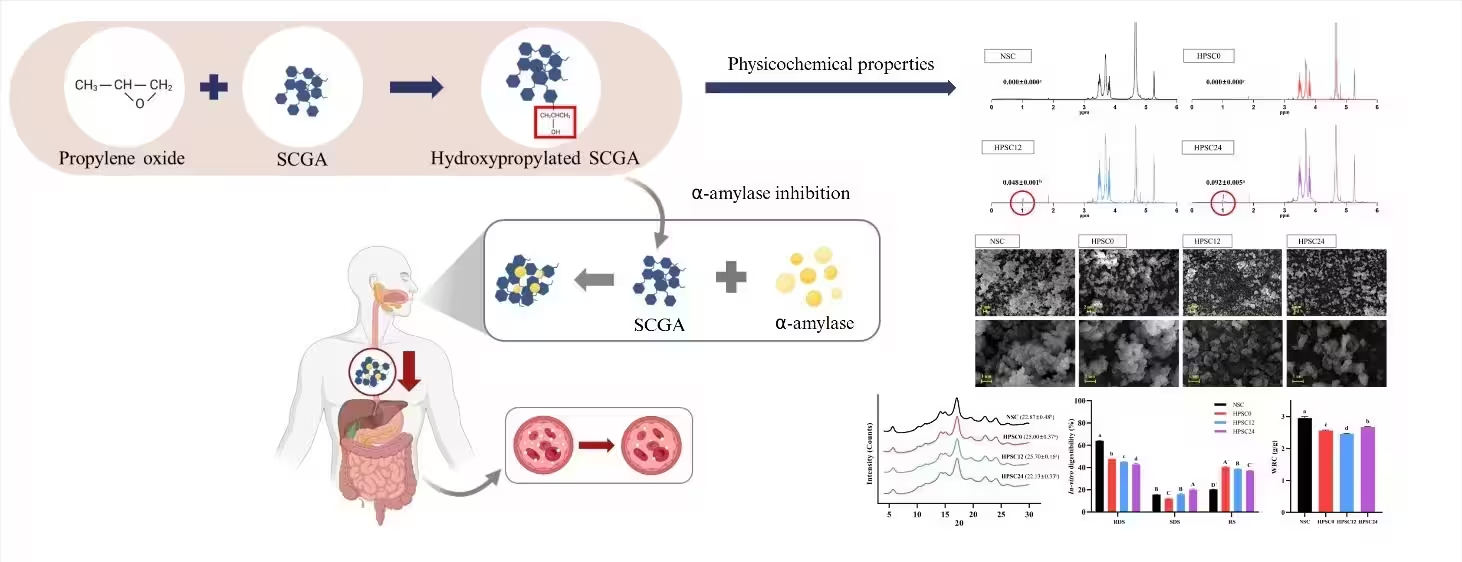
 Figure 1. Schematic diagram of Physicochemical properties of hydroxypropylated short-chain glucan aggregate
Figure 1. Schematic diagram of Physicochemical properties of hydroxypropylated short-chain glucan aggregate
Short-chain glucan aggregate (SCGA) is a starch nanoparticle formed by
self-assembly of short-chain glucans (SCGs) obtained through enzymatic
debranching of starch. It is a submicron-sized particle that functions as a
type of resistant starch and exhibits notable inhibitory effects on
α-amylase activity. Therefore, SCGA has been studied as a promising material
due to its potential to attenuate glycemic response. Hydroxypropylation (HP)
is a chemical modification method that hydroxypropyl groups replace the
hydroxyl groups of starch, thereby delaying starch retrogradation and
increasing paste transparency. HP increases resistant starch content and
enhances water retention, consequently inhibiting amylase activity. However,
there have been no reports on the properties of hydroxypropylated SCGA
(HPSC). Thus, this study aimed to investigate the physicochemical properties
of hydroxypropylated SCGA.
SCGA was produced by gelatinizing waxy corn starch, debranching it with
pullulanase, and self-assembling the resulting SCG. HP was carried out by
inducing an ether bond with 0 (HPSC0), 12% (HPSC12), and 24% (HPSC24)
propylene oxide at pH 11.5. The degree of substitution (DS), DSC, XRD, NMR,
SEM, in-vitro digestibility, and α-amylase inhibition of the
samples were analyzed.
Native SCGA (NSC) and HPSC0 showed no DS, whereas HPSC12 and HPSC24 revealed
DS values of 0.048 and 0.092, respectively. Annealing treatment under
alkaline conditions during hydroxypropylation prevented agglomeration of
SCGA particles. Moreover, the structural rearrangement induced by annealing
resulted in the highest enthalpy and relative crystallinity in HPSC0.
However, in HPSC24, the DS was sufficiently high to offset the effects of
annealing, leading to decreases in both enthalpy and relative crystallinity.
These results suggest that the introduced hydroxypropyl groups in HPSC24
interfered with double-helix formation and reduced the development of
crystalline structures. Water retention capacity decreased in HPSC0 because
alkaline treatment formed particles with a more ordered structure, but it
showed an increase after hydroxypropylation. For in-vitro
digestibility, all HPSC samples exhibited higher RS contents than NSC. HPSC0
showed the highest RS content, indicating that the structural reorganization
caused by annealing most effectively hindered enzymatic digestion. According
to a previous study, SCGA was reported to act as a competitive-type
inhibitor of α-amylase. HPSC24 demonstrated the strongest α-amylase
inhibitory effect, possibly due to their loosened double-helical structures
leading to not only compete with the substrate but also entrap α-amylase.
Overall, hydroxypropylated SCGA could enhance RS content and inhibit
α-amylase activity, due to alkaline annealing and their looser structure
formed by hydroxypropyl substitution, respectively. Hydroxypropylated SCGA
have potential as a material capable of lowering postprandial glycemic
response and can be utilized as a promising carrier for other bioactive
compounds.