INTRODUCTION
The key effects of polysaccharides in frozen desserts are “ice crystal
stabilization” and “shape retention”. In this study, these two effects of
tamarind seed gum (TSG), a polysaccharide first industrialized in Japan,
were investigated by comparing it with locust bean gum (LBG) and guar gum
(GG). In particular, the effect of TSG on the stabilization function of ice
crystals was quantitatively clarified.
METHODS
Frozen desserts were prepared using standard formulations containing
polysaccharides: TSG (0.30 wt%), LBG (0.30 wt%), GG (0.30 wt%), and a
combination of TSG (0.15 wt%) + LBG (0.15 wt%). To evaluate the shape
retention effect, the weight of melted product was measured after leaving
the samples at room temperature.
The morphology of ice crystals was evaluated using the analysis of curvature
distribution, following the method proposed by Matsukawa et al. (2024). In
this approach, a series of three consecutive points on the ice crystal
surface is selected at regular intervals, and the curvature at the central
point is calculated based on the radius of a circle passing through the
three points. Then, a curvature distribution is plotted using the calculated
curvature values.
The samples composed of 60 wt% sucrose and polysaccharide aqueous solutions
were frozen and stored at −28°C for either 3 or 14 days prior to
evaluation.
RESULTS
The weight of melt loss after leaving frozen desserts containing each
polysaccharide at room temperature for 60 minutes was measured in the
following increasing order: TSG+LBG < LBG < TSG < GG. The loss
weight of TSG+LBG sample was less than 10%, while that of other samples were
exceeded 80%. It was confirmed that the combined use of both TSG and LBG
dramatically improved the shape retention of frozen desserts.
The ice crystal morphology was also analyzed. The curvature distributions of
ice crystals for samples are shown in Fig. 1. For all samples, the curvature
distribution changed from 3 days to 14 days of storage: peaks in the
high-curvature range (reflecting round shapes) decreased, while peaks in the
low-curvature range (reflecting flat shapes) increased. This reflects the
visual change in ice crystal images which shows the amount of large and
flat-faced ice crystals was increasing over the frozen storage period. After
3 days of storage, differences between the added polysaccharide types were
not significant. However, after 14 days of storage, differences among each
polysaccharide became apparent. Compared to LBG and GG, TSG exhibited a
larger peak in the range of high curvature and a smaller peak in the range
of low curvature. It indicated that the addition of TSG restrained the
flattening of ice crystals. Furthermore, when TSG and LBG were combined, the
restraining effect was stronger than TSG. This suggests that the addition of
TSG, particularly the addition of combined TSG and LBG, suppressed changes
in ice crystal shape during the frozen storage period, thereby stabilizing
the ice crystals
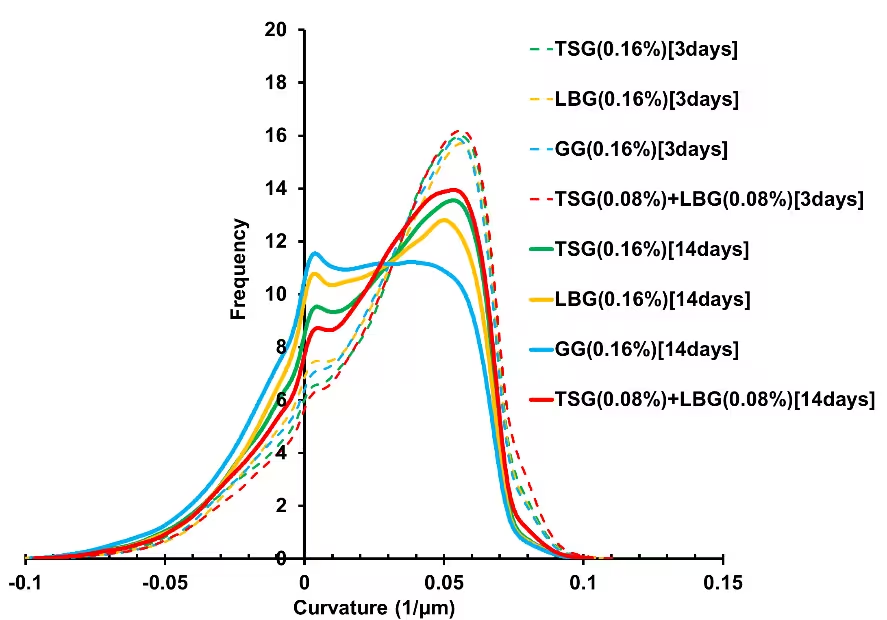 Fig. 1 Curvature distributions of ice crystals in 60% sucrose solution containing different polysaccharides after frozen storage for 3 and 14 days.
Fig. 1 Curvature distributions of ice crystals in 60% sucrose solution containing different polysaccharides after frozen storage for 3 and 14 days.
Acknowledgements
Authors wish to thank Professor Matsukawa of Tokyo University of Marine
Science and Technology for his valuable guidance.
Reference
[1] S. Ahmed, X. Yang, and S. Matsukawa, ‘A Novel Method for Analyzing the
Ice Crystal Shape from the Curvature of Ice Crystals’, Transactions of the
Japan Society of Refrigerating and Air Conditioning Engineers, 2024,
doi:10.11322/tjsrae.24-21
product development